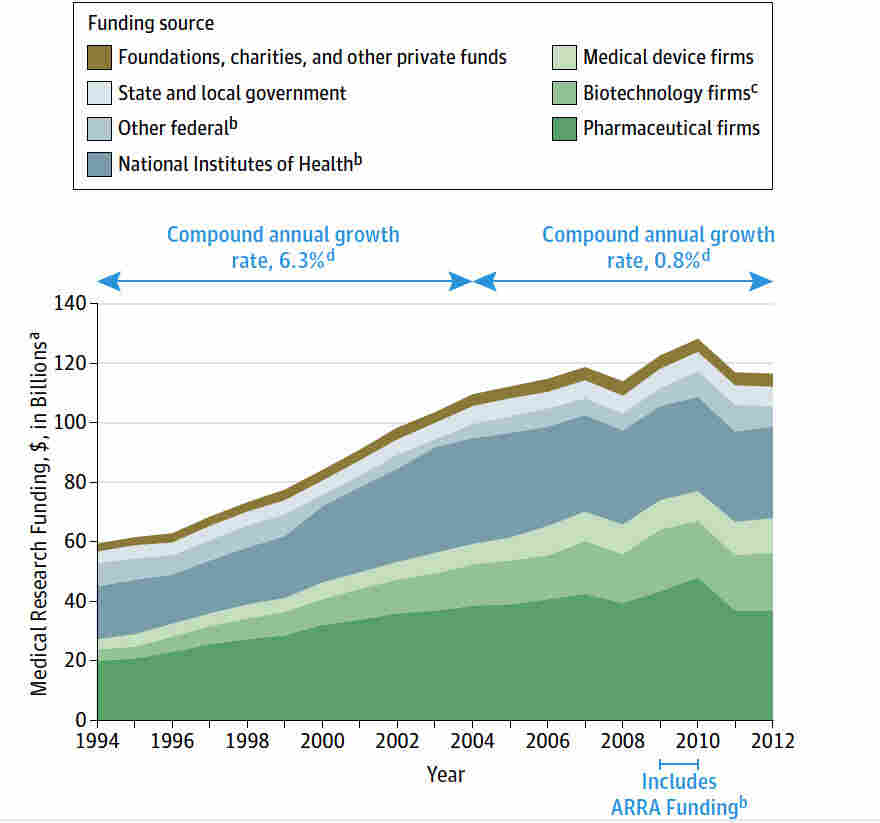
Medical research funding is crucial for advancing healthcare and ensuring patient safety in research. However, recent cuts in funding, such as the troubling NIH funding cuts, threaten vital projects that uphold rigorous IRB oversight essential for protecting participants in clinical trials. The impact of funding cuts can be detrimental, halting initiatives and casting doubt on the integrity of medical research processes. Institutions like Harvard, which rely on robust financial support, face significant hurdles in recruiting participants and validating their safety. Without adequate funding, the future of groundbreaking discoveries diminishes, putting the wellbeing of those involved in research at risk.
Securing financial resources for medical studies is paramount in the quest for better healthcare solutions. Investments in research are not just numbers; they represent hope for advancements in patient care and safety protocols. With alarming shifts in funding policies, the landscape for crucial initiatives is changing, particularly regarding oversight mechanisms like Institutional Review Boards (IRBs). As we engage in discussions around research finance, terms like clinical trial funding, research grants, and ethical oversight become increasingly relevant, interconnected through their collective influence on study integrity and participant welfare. Ensuring a steady influx of resources guarantees that researchers can focus on innovation while safeguarding the rights and safety of individuals involved in their work.
The Importance of Medical Research Funding
Medical research funding is crucial for the advancement of healthcare and the development of new therapies and treatment methods. A significant portion of this funding comes from the National Institutes of Health (NIH) and various federal grants, which facilitate groundbreaking studies that impact patient safety. When funding is abundant, research institutions can conduct comprehensive studies that not only improve treatment options but also ensure the safety and well-being of participants. However, when these funding streams experience cuts, as seen in recent federal funding freezes, it jeopardizes ongoing research efforts and puts patients at risk.
The reliance on consistent funding for medical research underscores the importance of investing in healthcare initiatives. With federal agencies like NIH facing budget cuts, researchers are often forced to halt projects, leading to gaps in knowledge that directly affect patient outcomes. Without adequate financial resources, institutions may struggle to meet ethical standards required for conducting medical research, ultimately hindering the ability to protect patients in clinical studies. As evidenced by the recent funding challenges faced by Harvard Medical School, such disruptions can lead to a substantial loss in progress towards patient safety and effective treatment development.
Impact of Funding Cuts on Patient Safety in Research
The impact of funding cuts on patient safety in medical research is profound and concerning. When research grants are limited, studies may not have the necessary resources to implement robust oversight mechanisms, such as Institutional Review Boards (IRBs). The disbanding or underfunding of these crucial oversight entities can lead to insufficient evaluation of research proposals, which in turn jeopardizes the health and safety of study participants. In the current landscape, a freeze on funding has resulted in a halt of ongoing IRB reviews, leaving many research proposals unassessed, ultimately increasing the risk of unintended harm to participants.
Moreover, funding cuts can create an environment of hesitation among researchers regarding the ethical implications of their work. With the essential support systems faltering, there exists a possibility that participants might not receive adequate information concerning risks and benefits associated with their involvement in studies. As historical precedents show, insufficient oversight can lead to exploitation and a breakdown of trust between participants and researchers. Thus, preserving funding for medical research programs and their requisite IRB oversight is vital to maintaining patient safety and encouraging public confidence in clinical research.
The Role of IRB Oversight in Protecting Patients
Institutional Review Boards (IRBs) serve as essential guardians of participant welfare in clinical research, ensuring that ethical standards are met and that participant safety is prioritized. These boards meticulously review research protocols to assess potential risks, the adequacy of informed consent processes, and the overall ethical considerations involved in the studies. IRB oversight not only protects individual rights but also reinforces public trust in medical research, highlighting the need for sufficient funding to sustain their operations. Without adequate financial backing, the efficacy of IRBs in protecting participants can be severely compromised.
The advent of guidelines that require single IRB review for multisite studies has further emphasized the necessity for efficient and knowledgeable IRB oversight. This streamlined approach is pivotal in rebuffing the delays and inefficiencies that can arise when multiple institutions conduct overlapping research. However, if funding cuts remove support from these institutions, it may result in weakened IRB capabilities, directly impacting how well they can protect patient participants from potential risks associated with clinical trials.
Federal Funding Cuts and Their Consequences
The recent federal funding cuts, particularly those impacting prominent research institutions like Harvard, raise critical concerns about the future of medical research and patient protection. As funding dries up, the ability of these institutions to carry out significant studies diminishes, placing a strain on the very infrastructure that is supposed to ensure participant safety. For example, studies that seek to explore new treatments for serious illnesses may face substantial delays or even cancellations, preventing the development of life-saving therapeutics.
Furthermore, the ripple effects of these funding cuts can lead to a broader public skepticism about the integrity of medical research. When studies are halted midway or experience significant disruptions, it may reinforce community mistrust among potential research participants. This mistrust can deter individuals from volunteering for future studies, reducing the diversity of the participant pool and thereby impacting the quality of research outcomes. Safeguarding research funding is not just a matter of maintaining scientific progress; it is vital for fostering an environment conducive to patient safety and trust.
Historical Lessons on Ethical Standards in Research
Reflecting on historical cases of medical misconduct, it is evident that robust ethical standards and oversight, driven by adequate funding, are paramount in ensuring patient safety in research. Events such as the Tuskegee Syphilis Study and the unethical hepatitis experiments at Willowbrook serve as stark reminders of what can occur in the absence of proper protections and oversight mechanisms. The establishment of IRBs was a response to these failures, emphasizing the critical nature of participant welfare and informed consent.
These historical lessons underscore the necessity for sustained investment in medical research funding, which not only supports the ethical oversight of studies but also empowers researchers to uphold the highest standards of responsibility. With a clear focus on protecting patients, the research community must remain vigilant against complacency. Funding cuts that diminish the oversight capabilities of IRBs threaten to unsettle the ethical foundations built upon countless lessons learned from the past.
Targeting Funding Towards Collaborative Research and Partnerships
Collaborative research has become increasingly essential in advancing medical science, particularly when addressing complex diseases that require interdisciplinary approaches. However, the success of these collaborations often hinges on adequate funding that supports multi-institutional projects and partnerships. By pooling resources and expertise, research institutions can tackle pressing healthcare challenges more effectively, ensuring a comprehensive focus on patient safety throughout the research process.
The role of funding is crucial in enabling institutions to streamline their research efforts while adhering to stringent IRB protocols. Collaborative initiatives can significantly enhance the scope of research, but they require coordinated support. As federal funding cuts continue to pose challenges, fostering a culture of collaboration may become increasingly difficult, potentially stalling advancements in medical research and putting patient safety at risk. Investing in funding for collaborative research is therefore imperative to ensure that innovations continue to emerge and patient safety remains a top priority.
The Future of Harvard Medical Research Amid Funding Challenges
Harvard Medical School has long been at the forefront of medical research, driving innovation and patient safety through rigorous scientific inquiry. However, recent funding cuts have cast a shadow on this legacy, raising concerns about the institution’s ability to maintain its commitment to ethical research practices. As federal grants become scarcer, Harvard, like many other research institutions, faces significant challenges in meeting the essential oversight requirements demanded by IRBs.
The future of Harvard’s research endeavors largely depends on its ability to navigate these funding hurdles while continuing to uphold the highest ethical standards. Support from institutional sources may temporarily alleviate some of the adverse effects of funding interruptions, but long-term sustainability hinges on reinstating prior levels of federal support. The ability to conduct groundbreaking studies, which not only advance medical knowledge but also prioritize patient safety, must take precedence moving forward.
Building Public Trust Through Ethical Research Practices
Establishing and maintaining public trust is a cornerstone of ethical medical research, underscoring the importance of transparency and integrity in all processes. When funding cuts lead to inadequate oversight or ethical lapses, it not only affects the immediate research but also has lasting implications for public perception of the research community as a whole. Stakeholders—including healthcare providers, researchers, and participants—must work collectively to foster an environment where ethical practices are the norm, ensuring that participant safety is always prioritized.
Communities must be actively engaged in conversations about the importance of medical research funding and the role it plays in their health. By fostering open dialogues that highlight the significance of ethical standards and government support in medical research, institutions can mitigate the distrust that emerges from funding cuts. Public trust is not built overnight; it requires consistent commitment to ethical practices, safeguarding participant welfare, and a willingness to communicate the critical importance of funding for sustaining these efforts.
The Broader Implications of Medical Research on Community Health
The impact of effective medical research extends far beyond individual studies, influencing community health on a much larger scale. When research initiatives are adequately funded, they yield powerful insights that can lead to significant public health improvements. The consequences of insufficient funding not only endanger specific patient populations but can also have adverse effects on community health as a whole. By neglecting research funding, we risk losing valuable opportunities to develop groundbreaking therapies and address pressing health challenges.
Moreover, the integration of community feedback into research processes fosters a sense of ownership and relevance among participants. When communities see their needs reflected in research priorities, trust grows, and participation becomes more prevalent. Medical research funding plays a critical role in ensuring studies not only align with participant needs but also result in tangible benefits for public health. Thus, prioritizing investment in medical research funding is essential for realizing better health outcomes for all.
Frequently Asked Questions
How do NIH funding cuts impact patient safety in medical research?
NIH funding cuts can severely disrupt the oversight of clinical trials and research studies aimed at protecting patient safety. When research grants are halted, it can lead to delays in Institutional Review Board (IRB) approvals, increasing the risk that studies do not adequately safeguard participants’ rights and welfare. Ensuring patient safety relies heavily on consistent funding for that regulatory oversight, which is essential for maintaining ethical standards in research.
What is the role of IRB oversight in relation to medical research funding?
IRB oversight plays a crucial role in medical research funding by reviewing research proposals to ensure the ethical treatment and safety of participants. When funding for research is cut, IRB processes may be delayed, which can hinder the initiation and progression of studies. Therefore, consistent medical research funding is vital to enable timely and thorough IRB reviews, ensuring continuous protection for research subjects.
What are the consequences of funding cuts in Harvard medical research?
Funding cuts in Harvard medical research, particularly large grants from NIH, can lead to project delays and disruptions across multiple clinical research studies. This compromises the capacity of researchers to innovate and adhere to rigorous ethical standards. The impact is not just on the researchers but also on patients involved in trials, whose safety and well-being may be compromised in a stalled research environment.
How does the impact of funding cuts affect the integrity of medical research?
Funding cuts can undermine the integrity of medical research by reducing the ability of researchers to carry out essential oversight and participant safety measures. Without proper funding, IRBs may be overwhelmed, causing ethical breaches and failing to protect study participants. Therefore, maintaining robust medical research funding is fundamental to ensuring research integrity and participant trust.
What historical events led to the need for improved medical research funding and oversight?
Historical events such as the unethical Tuskegee Syphilis Study and experiments during World War II highlighted the dire need for robust oversight in medical research. These incidents spurred the establishment of strict IRB regulations and increased funding for ethical oversight processes, reinforcing the importance of funding allocations that support patient safety and research integrity.
In what ways can medical research funding cuts increase public skepticism?
Medical research funding cuts can exacerbate public skepticism by halting ongoing studies and reducing transparency in research practices. When studies are delayed or disrupted, it breeds mistrust between the public and research institutions, making individuals less likely to participate in clinical trials and believe in the research outcomes. This cycle can undermine future funding opportunities and hamper scientific advancement.
How does funding from NIH support the ethical oversight of medical research?
NIH funding is critical for supporting the ethical oversight of medical research, as it provides the necessary resources for IRBs to operate effectively. These funds ensure that research proposals undergo rigorous ethical reviews before advancing. Continuous funding helps maintain safeguards for participant rights and promotes the responsible conduct of clinical trials.
What steps are being taken to address the impact of funding cuts on medical research?
Efforts to address the impact of funding cuts on medical research include advocacy for increased federal funding, collaboration between institutions to share resources, and the establishment of emergency funds to support ongoing studies. Institutions like Harvard Medical School are working to maintain collaborative research initiatives that ensure patient safety despite financial constraints.
Why is continued medical research funding essential for advancing healthcare?
Continued medical research funding is essential for advancing healthcare because it enables the exploration of new treatments, therapies, and safety protocols that benefit patients. Sufficient funding allows for the thorough review and monitoring of clinical trials by IRBs, ensuring the ethics and safety of research practices while fostering public trust in medical advancements.
| Key Issue | Description |
|---|---|
| Funding Freeze | The Trump administration froze over $2 billion in federal research grants to Harvard, disrupting critical medical research oversight. |
| SMART IRB | This system ensures oversight of multi-site medical studies, crucial for patient safety. |
| IRB Role | IRBs review and oversee research to protect participant rights, including risk assessment and informed consent processes. |
| Impacts of Funding Cuts | Funding cuts risk halting studies midstream, affecting participant safety and public trust in medical research. |
Summary
Medical research funding is crucial for safeguarding patient rights and ensuring ethical oversight in clinical studies. The recent halt in funding not only disrupts ongoing research but also poses risks to participant safety and public trust in medical innovation. Without adequate resources, systems like the SMART IRB, which provide essential oversight for multi-site studies, may falter, jeopardizing both research advancements and community health.