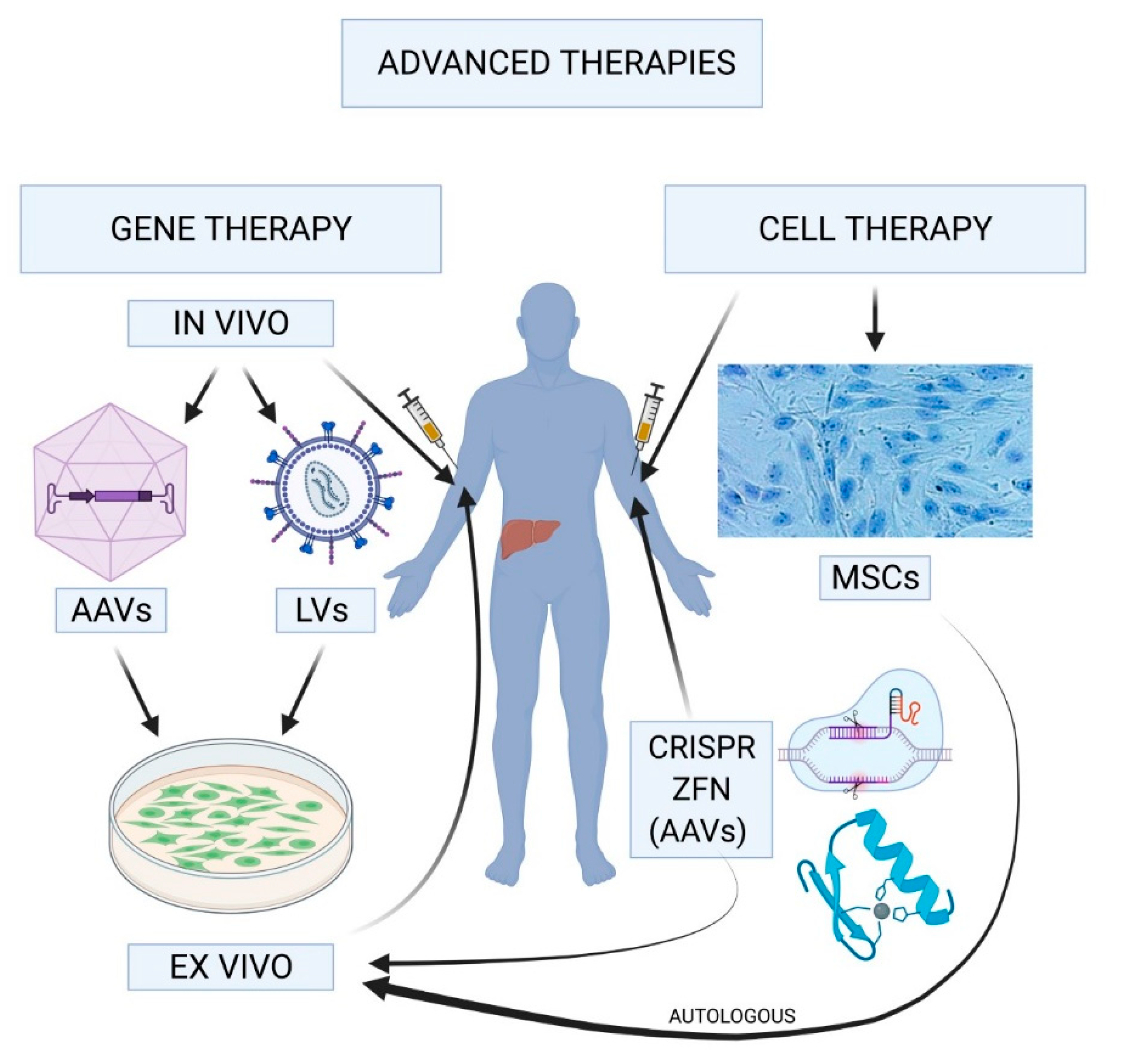
Hemophilia gene therapy is revolutionizing how we approach hemophilia treatment, offering new hope to those affected by this genetic disorder. This innovative gene therapy for hemophilia B, known as Hemgenix, has shown remarkable effectiveness in clinical trials, providing a potential cure rather than merely managing symptoms. For many, the prospect of life without constant needles and the anxiety of spontaneous bleeds is a dream becoming reality. As these new treatments for hemophilia emerge, they are set to transform hemophilia management, providing patients with a chance for a more normal, active lifestyle. With ongoing advancements in biotechnology, the future of hemophilia care may be brighter than ever before.
Gene therapy for hemophilia represents a groundbreaking shift in treating this hereditary bleeding disorder, targeting the root cause rather than just alleviating symptoms. Known as gene editing or genetic modification, these cutting-edge approaches like Hemgenix focus on providing sustainable solutions for individuals suffering from hemophilia B. Such therapies are at the forefront of modern medical science, aligning with the growing trend towards personalized medicine aimed at improving the quality of life for patients. The promise of these new innovations heralds a new era in hematology, where chronic conditions are managed with unparalleled effectiveness. As researchers explore the dynamics of gene replacement techniques and their long-term implications, the landscape of hemophilia treatment is poised for significant evolution.
Understanding Hemophilia and Its Challenges
Hemophilia is a genetic disorder characterized by the inability to properly clot blood due to a deficiency in certain clotting factors. The most common forms are hemophilia A and hemophilia B, with the latter being primarily caused by a deficiency of factor IX. This condition primarily affects males, as it is linked to the X chromosome. Living with hemophilia means that individuals must be vigilant about their activities to avoid injuries that could trigger potentially life-threatening bleeding episodes. Until recently, treatment mainly involved regular injections of clotting factors, which many patients, including Terence Blue, have had to endure for years.
Despite advancements in hemophilia management over the decades, such as the development of synthetic clotting factors that eliminate certain health risks, the traditional treatment protocols require constant medical attention and a stringent routine. For patients, this reality often leads to a lifetime burden of needles and the psychological toll of fearing the next spontaneous bleed. Moreover, the nature of hemophilia means that simple activities can carry significant risks, causing both physical limitations and social challenges.
The Evolution of Hemophilia Treatment Options
In recent years, the landscape of hemophilia treatment has seen dramatic shifts with the introduction of gene therapy, specifically tailored for conditions like hemophilia B. Gene therapy for hemophilia B, such as Hemgenix, represents a breakthrough that aims to modify the underlying genetic causes of the disorder. Instead of relying on daily injections of clotting factors, gene therapy offers the potential for long-term solutions by providing patients with a functional copy of the gene responsible for producing the missing clotting factor. For instance, Terence Blue, who received Hemgenix, reported significant improvements that highlight the therapy’s effectiveness in managing hemophilia.
The FDA’s approval of Hemgenix has been received with optimism not only among patients but also among medical professionals and researchers. This gene therapy approach is at the forefront of modern medicine, promising a shift in treatment paradigms from conventional methods that require frequent dosing to potentially curative options. The hope is that with ongoing advancements in gene therapy, future treatments could completely eliminate the need for factor replacement therapy, fundamentally transforming hemophilia management and improving the quality of life for those affected.
Exploring the Effectiveness of Hemgenix
Hemgenix, developed by CSL Behring, has shown remarkable effectiveness in clinical trials, with reports indicating that the majority of patients maintain sufficient factor IX levels long after treatment. For example, clinical data suggested that 94% of participants in the trials still did not require factor IX prophylaxis three years post-treatment. This impressive statistic signifies a potential turning point in hemophilia care, as patients can experience relief from the constant burden of treatment regimens, allowing them to engage more freely in everyday activities.
Moreover, the therapeutic mechanism of Hemgenix targets specific liver cells to induce the production of the clotting factor, which can significantly reduce the occurrence of bleeding episodes. This targeted approach is more effective than traditional methods, which often result in variable factor levels depending on how well the individual responds to injected clotting factors. As more patients gain access to gene therapy, the long-term benefits observed in studies will likely lead to changes in treatment guidelines and patient expectations surrounding hemophilia management.
Financial Considerations of Gene Therapy
While gene therapy holds great promise for hemophilia management, financial considerations remain a significant hurdle. Treatments like Hemgenix, although potentially curative, come with exorbitant price tags, often exceeding $3 million. This high cost reflects the complexities and lengthy processes involved in developing gene therapies. Insurers have been cautious in covering such treatments, leading to discussions about market access and the long-term sustainability of these advanced therapies. Patients and healthcare providers must navigate this challenging landscape as they seek to make informed decisions about treatment options.
As even some of the earlier gene therapies, like Pfizer’s Beqvez, have been withdrawn from the market due to lack of adoption and cost issues, it is vital for healthcare systems to establish frameworks that support patient access to innovative treatments. Ongoing dialogue among stakeholders, including pharmaceutical companies, insurance providers, and patient advocacy groups, is essential to address these financial barriers. Ensuring that new treatments become widely available could change the lives of many with hemophilia, moving them closer to a life free from the daily burdens of their condition.
Patient Experiences and Expectations with Gene Therapy
For patients like Terence Blue, the possibility of receiving gene therapy has been both liberating and daunting. Blue’s transition from traditional factor replacement therapy to Hemgenix represents a monumental shift in his life, where the prospect of alleviating the ongoing need for needles has ignited hope for many others in similar situations. The emotional impact of this shift cannot be understated; the potential to lead a life less constrained by bleeding episodes and treatments can transform not just health, but also one’s daily social interactions and overall quality of life.
However, patients have varied experiences and expectations regarding the new treatments. While some may find the breakthroughs thrilling, there is also a degree of apprehension about the long-term efficacy and potential side effects of gene therapy. Blue himself expressed cautious optimism, noting the tangible changes in his body’s ability to heal after receiving the therapy. His journey reflects not just the physical rehabilitation expected from innovative treatments, but the emotional landscape patients navigate as they grapple with their conditions and the hope that modern medicine provides.
Comparing Traditional Hemophilia Management to Gene Therapy
For decades, hemophilia treatment involved regular infusions of clotting factors, which, while effective, required a diligent routine that affected patients’ lifestyles. Traditional hemophilia management typically means frequent hospital visits, ongoing training for self-administration of injections, and a constant awareness of potential bleeding scenarios. Patients often need to carry emergency doses of clotting factors with them to prevent serious complications in case of injuries. This reality shaped the daily lives of many individuals living with hemophilia, forcing them to maintain a cautious approach to physical activities and social engagements.
In contrast, gene therapy for hemophilia B introduces a revolutionary paradigm that aims to free patients from the constraints of frequent treatment schedules. As seen with Hemgenix, patients may only require a single treatment that could offer long-lasting results in maintaining clotting factor levels. This evolution in treatment necessitates a complete reevaluation of how hemophilia is managed, shifting the focus from acute care interventions to more proactive and perhaps preventive measures. The potential of gene therapy to minimize the risks associated with spontaneous bleeds fundamentally alters the expectations and lifestyle of those diagnosed with hemophilia.
The Future of Hemophilia Research and Development
The field of hemophilia research is rapidly advancing, with innovative therapies such as gene therapy now changing how the medical community views this disorder. Researchers are optimistic that ongoing studies will lead to further breakthroughs and more effective treatment options for hemophilia and other genetic disorders. With the success of products like Hemgenix, the outlook for gene therapy as a viable long-term solution is promising. As more therapies become available, patient outcomes and quality of life are expected to improve significantly.
Moreover, collaborations among academic institutions, biotech companies, and healthcare providers are likely to foster a more robust ecosystem for hemophilia treatments. This synergy may also drive down costs over time, making these transformative therapies accessible to a broader patient population. As new treatments emerge from the pipeline, the continued investment in research and development will be essential to realizing the full potential of gene therapies, ensuring that patients are at the forefront of these medical advancements.
Living with Hemophilia: Beyond the Medical Perspective
While the medical advancements in hemophilia treatment are groundbreaking, it’s essential to consider the broader psychosocial implications of living with the condition. The daily management of hemophilia can lead to psychological strains such as anxiety and stress associated with the fear of bleeding episodes and their impact on activities. Community support and open discussions about the challenges faced by those living with hemophilia play a crucial role in mental wellness. Programs aimed at education and social integration can help patients lead fulfilling lives despite their condition.
Furthermore, as gene therapies like Hemgenix gain traction, the stigma surrounding hemophilia may diminish, allowing individuals to engage more fully in society. The stories of patients who successfully navigate their diagnosis offer hope and resilience to others. Awareness campaigns can elevate understanding among the general public, fostering a supportive environment that can improve the overall experience for those living with hemophilia. Society’s acceptance bolsters the need for robust healthcare policies that prioritize access to innovative treatments, ensuring that people with hemophilia have the opportunity to thrive.
Navigating Insurance Challenges for Gene Therapies
As hemophilia treatments progress towards advanced gene therapies, patients and healthcare providers face a complex landscape of insurance coverage and reimbursement issues. Many insurance plans remain uncertain about how to approach the high costs associated with gene therapies like Hemgenix. The reluctance of insurers to provide comprehensive coverage can lead to life-altering decisions for patients who may find themselves unable to afford their recommended treatment. While the potential for these therapies to significantly reduce long-term healthcare expenditures is evident, immediate access remains an obstacle.
Moreover, as patients advocate for broader access to life-altering treatments, navigating the insurance landscape becomes a critical aspect of hemophilia management. Patients must equip themselves with knowledge about their insurance policies and stay informed about legislative movements that may affect coverage of gene therapies. With the push for policy changes, the dialogue surrounding insurance coverage for innovative treatments is becoming increasingly important, highlighting the need for advocacy in ensuring that all patients can benefit from advancements in hemophilia care.
Frequently Asked Questions
What is hemophilia gene therapy and how does it work?
Hemophilia gene therapy is a novel treatment approach aimed at correcting the underlying genetic defect responsible for hemophilia, specifically hemophilia B. This therapy uses bioengineered viruses to deliver a normal copy of the gene responsible for producing clotting factor IX to the liver, enabling the body to produce the necessary clotting factors naturally, thus reducing the need for regular factor IX injections.
How effective is Hemgenix in treating hemophilia B?
Hemgenix has shown promising effectiveness in treating hemophilia B. Clinical trials indicate that up to 94% of patients treated with Hemgenix did not require factor IX prophylaxis three years after treatment, demonstrating the potential of this gene therapy to significantly improve the management of hemophilia.
What are the benefits of using gene therapy for hemophilia treatment?
Gene therapy offers several benefits for hemophilia treatment, including the potential for long-lasting effects with a single dose, reduced need for frequent injections of clotting factors, and improved quality of life for patients by minimizing the risk of bleeding episodes, thus allowing for more active lifestyles.
Are there any risks associated with hemophilia gene therapy?
While hemophilia gene therapy, such as Hemgenix, is generally considered safe, there are some associated risks. These can include potential liver enzyme elevation, immune responses to the viral vector used in therapy, and the need for close monitoring post-treatment. It is essential for patients to discuss these risks with their healthcare providers.
What advancements in hemophilia management does Hemgenix represent?
Hemgenix represents a significant advancement in hemophilia management by providing a potentially curative treatment option for hemophilia B patients. Unlike traditional treatments that typically require lifelong regular infusions of clotting factors, Hemgenix offers a one-time gene replacement approach, which could transform the landscape of hemophilia treatment.
How can patients access hemophilia gene therapy like Hemgenix?
Patients interested in accessing hemophilia gene therapy, such as Hemgenix, should consult with their healthcare providers to evaluate their eligibility. Most therapies are administered in specialized medical centers that have experience with gene therapy treatments and the necessary protocols in place for patient care and monitoring.
What is the cost of hemophilia gene therapies, particularly Hemgenix?
The cost of hemophilia gene therapies like Hemgenix can be substantial, with a list price of approximately $3.5 million. However, many patients may benefit from insurance negotiations or assistance programs, which can help mitigate the financial burden associated with these innovative treatments.
What is the future outlook for new treatments for hemophilia?
The future outlook for new treatments for hemophilia is optimistic, as ongoing research and clinical trials continue to explore and develop advanced gene therapies and other novel approaches. The potential for transformative therapies is expanding, with the aim of enhancing the management and quality of life for patients with hemophilia.
| Key Points | Details |
|---|---|
| Patient’s Experience | Terence Blue underwent gene therapy for hemophilia B, claiming he is healing faster than ever. |
| Gene Therapy Introduction | Hemgenix, developed by CSL Behring, was FDA-approved in November 2022 for hemophilia B. |
| Historical Context | Blue has managed hemophilia from a young age, receiving injections regularly until gene therapy was available. |
| Market Challenges | High costs and market pressures lead some therapies to be withdrawn despite clinical potential. |
| Experimental therapy | Hemgenix uses a virus to correct mutations in liver cells to produce clotting factor IX. |
| Efficiency and Results | Long-term studies suggest that the majority of patients may not need prophylactic treatment post-therapy. |
| Future Implications | Gene therapy may revolutionize treatment for hemophilia, with higher life quality and fewer injections. |
Summary
Hemophilia gene therapy offers promising new prospects for patients like Terence Blue, who is experiencing rapid healing post-treatment. This innovative approach, represented by therapies such as Hemgenix, provides long-awaited relief from the burdens of constant injections and bleeding management. As advancements continue, gene therapy shines as a beacon of hope for many individuals suffering from hemophilia, potentially transforming their lives and reducing dependence on traditional treatments.