Patient safety in medical research is an indispensable cornerstone of ethical scientific inquiry, ensuring that individuals participating in clinical trials are protected from harm. As funding for medical research becomes increasingly scrutinized, particularly federal grants such as those from NIH, the oversight mechanisms essential for maintaining these standards may be jeopardized. Institutions rely on rigorous institutional review boards (IRBs) to navigate the complexities of research ethics and regulations, safeguarding patients’ rights and welfare across studies. However, recent disruptions in medical research funding have put significant strains on these oversight processes, complicating clinical research oversight at universities and hospitals nationwide. The implications of these cuts not only threaten the integrity of research but ultimately risk the well-being of the very patients these studies aim to benefit.
Ensuring the safety of individuals involved in clinical studies is vital in the landscape of health research. When exploring medical investigations, one must consider the critical role of ethical committees that monitor the well-being of participants, safeguarding their interests throughout the research process. With financial backing from government bodies, such as the National Institutes of Health (NIH), researchers can conduct their work under stringent ethical guidelines and oversight regulations. Yet, emerging challenges like funding freezes highlight the fragility of these support systems and their impact on the essential safety measures for patients. As the discourse on research ethics evolves, it becomes clearer that robust funding and governance structures are crucial for protecting individuals who volunteer for medical trials.
The Importance of Patient Safety in Medical Research
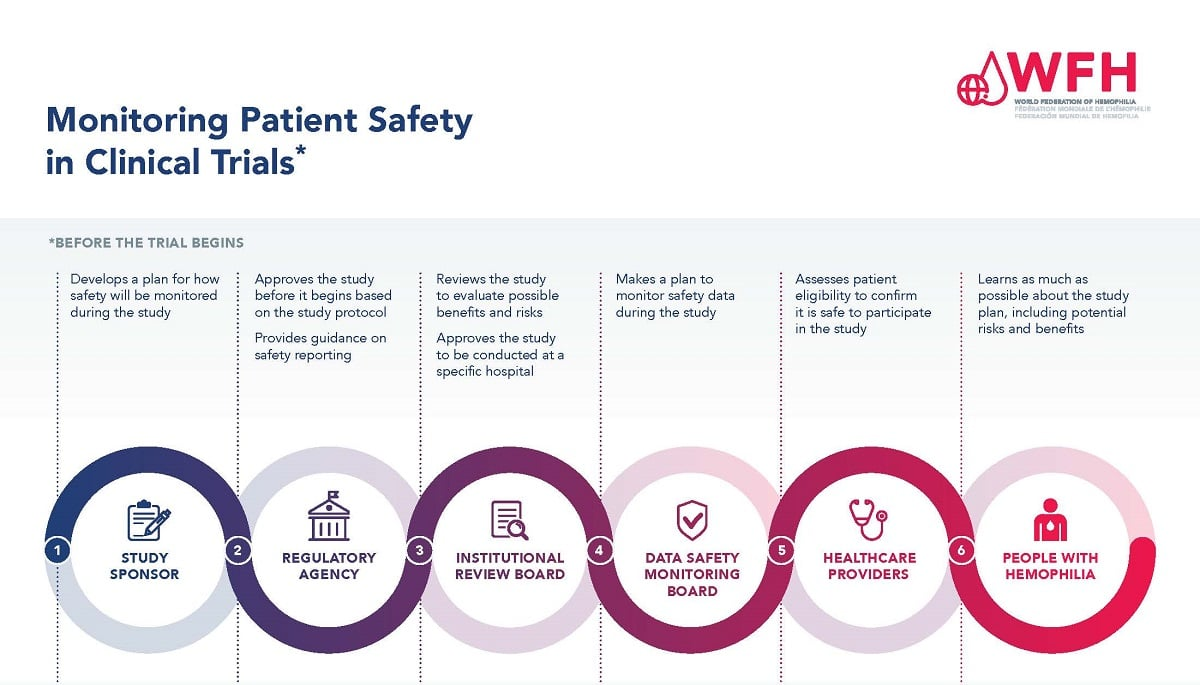
Patient safety is the cornerstone of medical research, ensuring that participants are protected from potential harm during clinical trials. With the increasing complexity of research protocols and the variability in individual responses to medical interventions, organizations have recognized the necessity of robust oversight mechanisms to safeguard participants’ rights and welfare. Institutional Review Boards (IRBs) play a pivotal role in this aspect, diligently reviewing the ethical considerations of studies before they are approved. By evaluating factors such as informed consent, risk assessment, and potential adverse effects, IRBs help bolster patient confidence in research practices.
As highlighted by the disruptions caused by funding freeze, the integrity of patient safety measures can be compromised when financial resources are cut. Reduced funding impacts not only the design and execution of studies but also hampers the ability of IRBs to perform necessary functions. Without adequate financial support, IRBs may struggle to maintain rigorous oversight, thereby threatening the safety of participants. This situation underscores the vital link between sustained funding and the ethical conduct of medical research, emphasizing that patient safety must not be sacrificed in the face of budgetary constraints.
Cutting Funding and Its Consequences for Clinical Research Oversight
The freeze on federal research grants, as experienced by Harvard and other institutions, has immediate and far-reaching consequences for clinical research oversight. Research funding is essential for the establishment and maintenance of infrastructures that monitor research compliance, ensuring that studies adhere to ethical standards and regulations. The inability to fund multiple research sites means that investigations into critical health issues, such as vaccine efficacy and cancer treatments, face delays or may be altogether abandoned. This not only hinders scientific innovation but also stalls advancements in patient care.
Moreover, the ripple effect of funding cuts extends beyond individual studies. It can diminish the training and support provided to researchers and IRB members, thereby eroding the overall quality of clinical research oversight. Established protocols for ethical review could falter without the required resources to implement them effectively. The implications are profound, as compromised oversight may lead to increased risks for study participants, ultimately undermining public trust in research institutions. The overarching message is clear: stable funding is crucial to uphold the integrity of medical research and protect those who participate.
Effects of NIH Funding on Research Ethics and Regulations
NIH funding has historically played a significant role in shaping the landscape of medical research ethics and regulations. Financial support from the NIH not only promotes innovative research but also ensures adherence to ethical standards that protect participants’ rights. By requiring specific protocols, such as the use of single IRBs for multi-site studies, the NIH has streamlined regulatory processes while emphasizing the importance of participant welfare. These supportive measures foster a culture of accountability and transparency in research, which is vital for maintaining public trust.
As NIH funding is cut, the risk to ethical oversight in research increases exponentially. Institutions may feel compelled to compromise on important ethical practices due to financial constraints, which can result in inadequate protection for research participants. Furthermore, the erosion of NIH-led initiatives might lead to disparate practices among research institutions, creating inconsistencies in how ethics are applied across studies. Such variations can weaken the regulatory landscape, place participants at risk, and ultimately compromise the validity of research results, hindering the quest for new medical breakthroughs.
IRB’s Role in Ensuring Ethical Research Practices
Institutional Review Boards (IRBs) are pivotal in the triage of ethical research practices, scrutinizing every proposal to ensure participants’ safety and rights are prioritized. Their comprehensive review processes address all aspects of a study, including consent procedures, risk management, and ethical treatment of subjects. In light of historical ethical breaches in research, such as the infamous Tuskegee study, the establishment of IRBs was a crucial step in instituting protective measures for human subjects. Today, they serve as the frontline guardians against potential research misconduct.
However, the efficacy of IRBs is intrinsically linked to the availability of sufficient funding and resources. When financial support is threatened, IRBs may struggle to operate efficiently, risking their ability to conduct thorough reviews. Inadequate oversight not only endangers participant safety but also undermines public confidence in research initiatives. Continuous education and training of IRB members, funded by grants and institutional support, are essential to adapt to evolving ethical standards and address the emerging complexities of modern medical research.
The Impact of Funding Cuts on Collaborative Research
In an era of rapidly advancing medical technologies and collaborative research initiatives, funding cuts present a severe impediment. Collaborative projects often involve multiple institutions pooling resources to address complex health challenges, such as chronic diseases or pandemics. Without sufficient funding, the infrastructure necessary for facilitating these collaborations is jeopardized, hindering researchers’ ability to share findings and best practices. This fragmentation can lead to delays in important research, ultimately affecting patient access to new treatments.
Furthermore, diminished collaboration due to funding constraints can translate into missed opportunities for innovative clinical trials that are essential for assessing new therapies. Research institutions and hospitals need to engage in synergy to leverage diverse expertise and resources effectively. The erosion of this cooperative spirit threatens to stall the progress made in addressing pressing health concerns, putting patients’ health at risk. Thus, the interdependence of funding stability and cooperative research is crucial for the continued advancement of medical science.
Strengthening Patient Trust through Ethical Oversight
Establishing and maintaining patient trust is foundational to the success of medical research. Patients must feel assured that their safety and well-being are prioritized throughout the research process. This is where the thorough oversight provided by IRBs and adherence to research ethics become indispensable. Ethical oversight fosters transparency, encouraging potential participants to engage in studies with confidence, knowing their interests will be protected. The relationship between researchers and communities can flourish when grounded on trust and shared ethical considerations.
Funding cuts can undermine this essential trust. When financial resources are limited, oversight bodies may struggle to fulfill their obligations, leading to a perception of compromised ethics in research. Over time, this could result in diminished participation in clinical trials, as potential volunteers may hesitate to engage in research that lacks robust oversight. Restoring and maintaining public trust through ethical and transparent practices is critical, emphasizing that patient safety in medical research should remain a non-negotiable priority, supported by sustained funding.
The Role of Research Ethics Training in Safeguarding Patients
The importance of research ethics training cannot be underestimated in the context of safeguarding patient safety during medical research. Training equips researchers, IRB members, and clinical staff with the knowledge and skills necessary to navigate the complex ethical landscape inherent in human studies. By emphasizing principles such as informed consent, risk assessment, and respect for persons, ethics training lays the foundation for responsible research conduct that prioritizes participant safety and welfare.
However, funding cuts can adversely affect the availability and quality of ethics training programs. With fewer resources, institutions may be unable to provide comprehensive training sessions or keep staff updated on current regulations and ethical standards. This can lead to lapses in ethical oversight, compromising participant safety and increasing the risk of misconduct in research. Investing in robust ethics training is essential to foster a culture of accountability and ensure that researchers are well-equipped to protect participants in their studies, highlighting the interconnectedness of funding, ethics, and patient safety.
Regulatory Compliance in Medical Research: A Critical Component
Regulatory compliance is a fundamental requirement for all medical research involving human participants. Adhering to established laws and ethical guidelines safeguards participants and maintains the integrity of scientific findings. Compliance encompasses various aspects, from securing informed consent to reporting adverse events and maintaining participant confidentiality. In addition, regulatory bodies, such as the FDA and NIH, provide essential oversight that contributes to the safety and ethical conduct of research studies.
Funding cuts, however, threaten researchers’ ability to maintain full compliance with regulatory requirements. Without adequate resources, institutions may struggle to support the administrative processes needed for compliance monitoring, risking potential violations that could endanger participants. Ongoing training, administrative costs, and infrastructure development are all essential components of regulatory compliance, and when funding becomes limited, the risk of lapses in compliance escalates, ultimately undermining patient safety and the credibility of medical research.
Future Directions: Advocating for Robust Funding Models
To ensure the continuation of safe and ethical medical research practices, there is an urgent need for advocacy around robust funding models. Proactive measures must be taken to secure consistent and dependable funding streams that support the infrastructure necessary for oversight, compliance, and ethical training. Collaborative efforts among universities, institutions, and policymakers can create frameworks that promote stable funding while emphasizing the importance of patient safety in research.
Future funding strategies should incorporate the lessons learned from past disruptions, ensuring that patient safety remains a priority regardless of funding climates. Continued investment in SWAT IRB systems and similar initiatives is vital for effective oversight of multi-site collaborations. By reinforcing the connection between funding, ethics, and patient safety, the research community can work together to create a resilient and trustworthy environment that prioritizes the welfare of research participants.
Frequently Asked Questions
How does NIH funding contribute to patient safety in medical research?
NIH funding is vital for ensuring patient safety in medical research as it mandates that studies involving human participants undergo rigorous review by Institutional Review Boards (IRBs). This approval process confirms that research complies with ethical standards, protecting the rights and welfare of participants. Moreover, NIH policies, particularly those requiring the use of single IRBs for multisite collaborations, streamline oversight and enhance safety measures across various research sites.
What is the role of IRBs and patient safety in medical research?
IRBs are crucial in maintaining patient safety in medical research. They evaluate research proposals to ensure that participants are not subjected to unnecessary risks and that informed consent processes are clear and ethical. By monitoring studies’ design and implementation, IRBs help mitigate potential harms, ensuring the protection of individuals involved in research endeavors.
How do funding cuts impact clinical research oversight and patient safety?
Funding cuts can severely disrupt clinical research oversight, leading to potential lapses in patient safety. When research projects face financial constraints, IRBs may struggle to maintain adequate oversight, increasing the risk of ethical breaches and harm to participants. Halting studies mid-course exacerbates these risks, as ongoing research may lack the necessary resources to safeguard participant welfare effectively.
What historical events influenced research ethics and patient safety regulations?
Several historical atrocities, such as the Tuskegee Syphilis Study and unethical medical experiments during World War II, catalyzed the establishment of stringent regulations overseeing patient safety in medical research. These events highlighted the necessity for robust Institutional Review Boards and the ethical conduct of researchers to ensure that the rights and welfare of participants are always protected.
How does a lack of research funding threaten patient safety in medical studies?
Reduced research funding can lead to compromised patient safety in medical studies by hindering the comprehensive oversight necessary for ethical research practices. With fewer financial resources, IRBs may be unable to conduct thorough reviews of studies, resulting in potential risks to participants and increasing public mistrust in medical research outcomes.
What measures exist to ensure patient safety in multi-site clinical research?
To ensure patient safety in multi-site clinical research, the NIH requires that studies use a single IRB for oversight. This approach simplifies compliance and enhances the coordination of safety measures across different participating institutions, providing a consistent framework for protecting research participants throughout the study.
What are the implications of delays in medical research on patient safety?
Delays in medical research can have serious implications for patient safety. When studies are postponed, participants may miss out on potential treatment options, and progress toward understanding diseases can stagnate. Research interruptions can also damage public trust, making it more challenging to recruit participants for future studies, which is critical for advancing medical knowledge and patient care.
How do community engagements enhance patient safety in medical research?
Community engagement plays a pivotal role in enhancing patient safety in medical research by fostering trust and transparency. When researchers work collaboratively with communities, it ensures that studies are designed with patient needs in mind, promoting ethical practices and support for volunteer participation. This collaborative approach also encourages diverse perspectives, improving the relevance and safety of research findings.
| Key Points |
|---|
| The Trump administration’s freeze of over $2 billion in federal research grants to Harvard has significantly affected patient safety in medical research. |
| SMART IRB is a national system designed to facilitate oversight of medical studies, ensuring compliance with ethical standards and protecting research participants. |
| Institutional Review Boards (IRBs) are essential in reviewing research proposals, assessing risks, and ensuring informed consent for research participants. |
| Funding cuts to research institutions could halt ongoing studies, increasing risks and undermining public trust in medical research. |
| Historical abuses in medical research highlight the importance of ethical oversight provided by IRBs. |
Summary
Patient safety in medical research is critically impacted by funding challenges and regulatory changes. The ongoing discussions around ethical oversight and the role of Institutional Review Boards (IRBs) reaffirm the necessity of maintaining diligent research practices that prioritize participant welfare. As funding cuts threaten vital research initiatives, it’s evident that safeguarding patient rights and safety remains paramount in advancing medical science. Addressing these issues is essential to restore trust and ensure that medical research continues to provide safe and effective innovations for public health.